Organic Chemistry
Introduction
Organic chemistry, also known as “carbon chemistry”, is chemistry involving organic — or “carbon-based” — molecules. All life on Earth can be considered an expression of complex carbon chemistry. However, since lots of non-living things are carbon-based as well, organic chemistry isn't merely limited to biochemistry. Organic chemistry also deals with polymers, petroleum products, and much more.
Organic Molecule Classes & Functional Groups
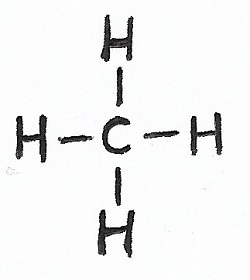 Methane |
Alkanes & Alkyl Groups — alkane molecules and alkyl functional groups are single-bonded hydrocarbons.
|
 Ethene |
Alkenes & Alkenyl Groups — characterized by double-bonds between carbons.
|
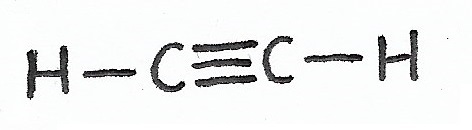 Ethyne |
Alkynes & Alkynyl Groups — alkyne molecules and functional groups have triple-bonds between carbon atoms. |
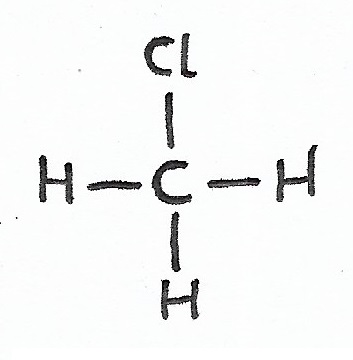 Chloromethane |
Alkyl Halides & Halide Groups — organic halide molecules, also known as halocarbons or alkyl halides, are carbon-based molecules that contain one or more halogens. |
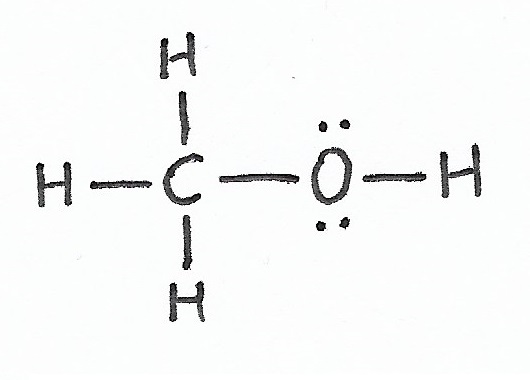 Methanol |
Alcohols & Hydroxyl Groups — alcohols are molecules that have one or more hydroxyl groups. |
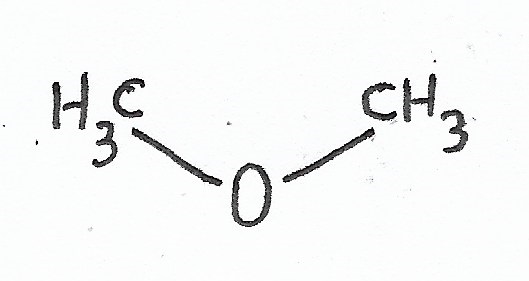 Dimethyl Ether |
Ethers — |
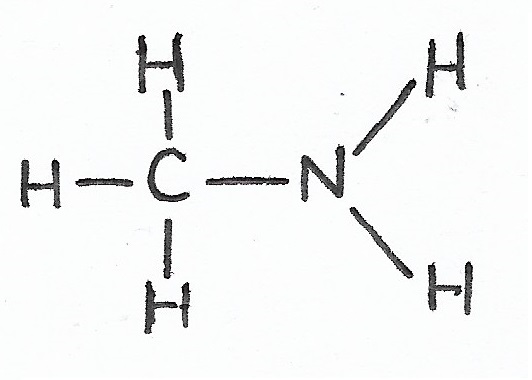 Methyl Amine |
Amines — |
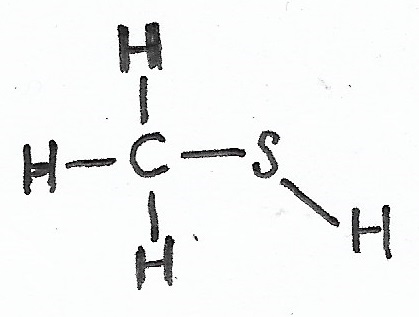 Methanethiol |
(Organic) Thiols — |
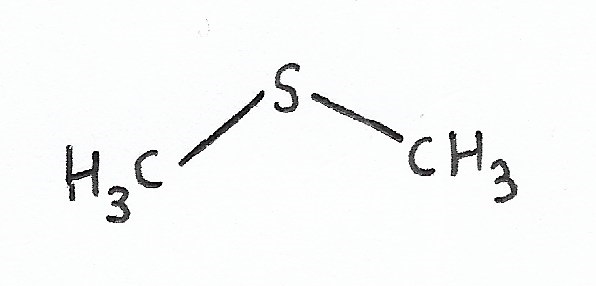 Dimethyl Sulphide |
(Organic) Sulphides — |
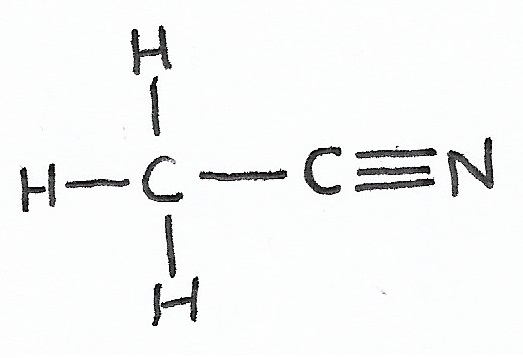 Acetonitrile |
Nitriles — |
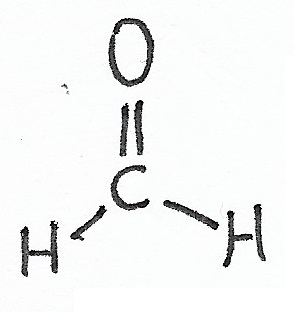 Methanal |
Aldehydes — the simplest aldehyde is methanal, also known as formaldehyde. |
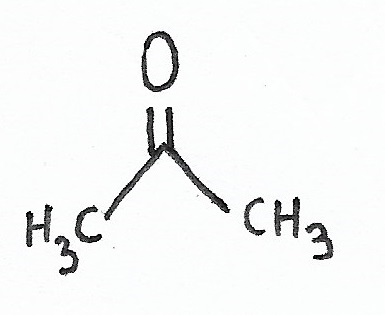 Propanone |
Ketones — the simplest ketone is propanone. |
 Methanoic Acid |
Carboxylic Acids — the simplest carboxylic acid is methanoic acid, also known as formic acid. |
Molecular Geometry
For more information, see molecular geometry.
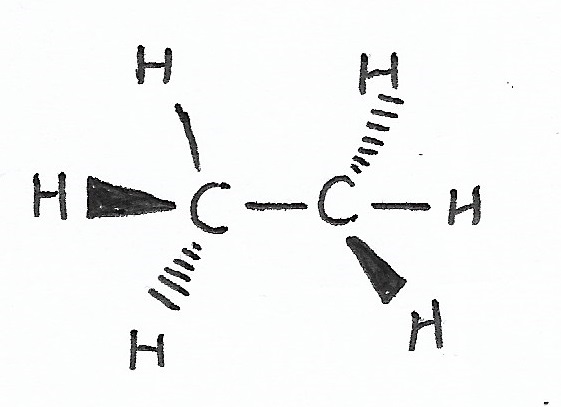 |
Orbital Hybridization — has to do with the angle of bonds to atoms. |
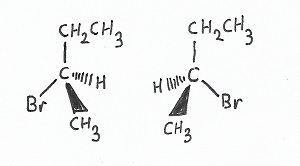 |
Chirality — has to do with the ordering of bonds to atoms. |
Conformation
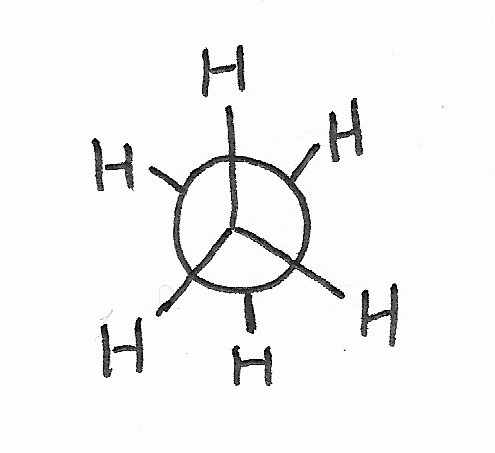 |
Staggered Conformation — is the stablest conformation for C-C bonds. |
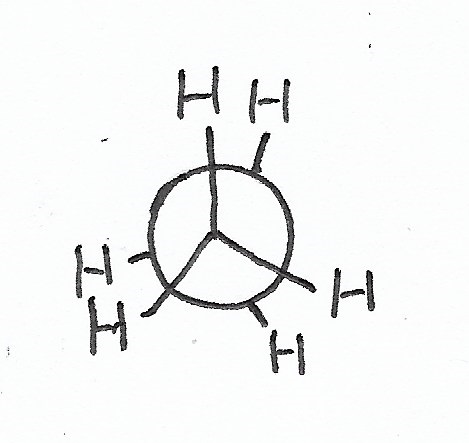 |
Eclipsed Conformation — causes the most steric hindrance. |
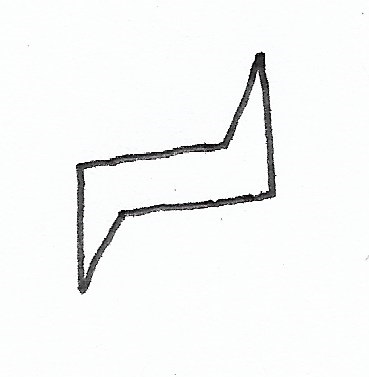 |
Chair Conformation — the stablest conformation for cyclohexane rings. |
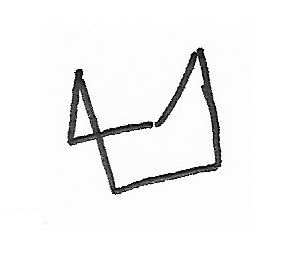 |
Boat Conformation — |
Isomerism
For more information, see isomer.
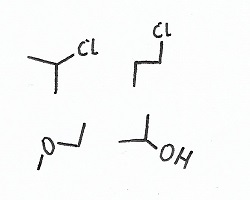 |
Constitutional Isomers — have the same chemical formula (i.e., the same number of every kind of atom) but are put together in entirely different ways. |
 |
Stereoisomers — have the all the same atoms bonded to the same atoms, thus having not only the same constitution but also somewhat similar configuration, however the bonds between some of these atoms are oriented differently. |
Ionization & Formal Charge
 |
Carbocation — a carbon with only three bonds and a positive formal charge. |
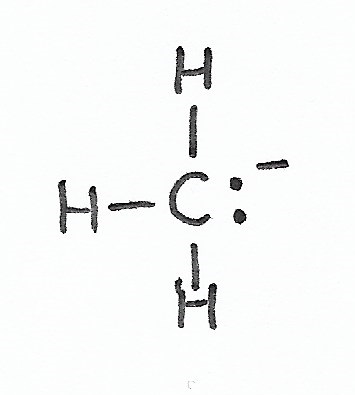 |
Carbanion — has one lone pair of valence electrons and a negative formal charge. |
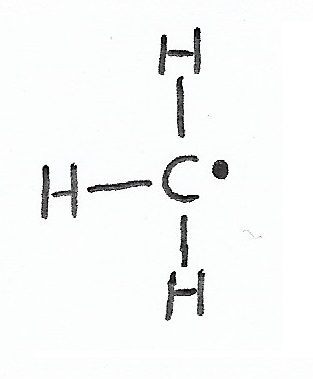 |
Free Radical — have a single, unpaired valence electron and a formal charge of zero. |
Acids & Bases
For more information, see acids & bases.
 |
Acid Disassociation Constant [Ka] — stronger acids have a larger Ka value. |
|
pKa — negative log of the acid disassociation constant. |
|
|
Proton Concentration [pH] — a lower pH value indicates greater acidity, while a higher pH value indicates great basicity. |
|
|
Equilibrium Constant [Keq] — |
Table of Reactions
| Reaction Type | Reagents | Products | ||||
| Addition | Polar | Electrophilic | Dihalo addition | |||
| Hydrohalogenation | ||||||
| Hydrogenation | ||||||
| Hydration | Hydroboration-Oxidation | Alkenes | Alcohols | |||
| Mukaiyama’s Hydration | ||||||
| Oxymercuration | ||||||
| Nucleophilic Addition | ||||||
| Non-Polar | Free-radical | |||||
| Cycloaddition | ||||||
| Substitution | Electrophilic | Aromatic | ||||
| Aliphatic | Nitrosation | |||||
| Ketone halogenation | ||||||
| Keto-enol tautomerism | ||||||
| Nucleophilic | Unimolecular [SN1] | |||||
| Bimolecular [SN2] | ||||||
| Aromatic [SNAr] | ||||||
| Internal [SNI] | ||||||
| Elimination | Unimolecular [E1] | |||||
| Bimolecular [E2] | ||||||
Regiochemistry
Markovnikov
Stereochemistry
Anti adddition
Syn addition
Erata
Check out this pure HTML periodic table of elements.

