Chemistry
Introduction
The exact dividing line between physics and chemistry is difficult, if not impossible, to establish. This is partly because physics deals with interactions between objects and forces at the largest and smallest scales of reality, while chemistry deals with interactions at a level just above and slightly overlapping the scale of particle physics (notably, both particle physics and chemistry deal with interactions between protons, neutrons, electrons, and atoms). Indeed, just as biology could be described as an expression of complex chemistry, chemistry could be described as an expression complex particle physics. If not for the fact that chemistry is an older science than physics, it probably would have been classified as a subdiscipline of physics.
Check out this pure HTML periodic table of elements.
Types of Chemistry
|
Analytical Chemistry — deals with the recognition/detection of chemicals. |
|
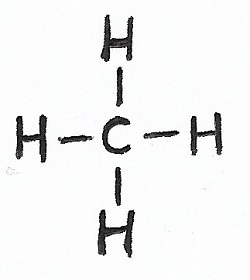 |
Organic Chemistry — also known as “carbon chemistry”, is chemistry involving organic — or “carbon-based” — molecules. All life on Earth can be considered an expression of complex carbon chemistry. However, since lots of non-living things are carbon-based as well, organic chemistry isn't merely limited to biochemistry. Organic chemistry also deals with polymers, petroleum products, and much more. |
|
Inorganic Chemistry — |
|
|
Physical Chemistry — |
|
|
Plasma Chemistry — |
|
Acids & Bases
For more information, see acids & bases.
 |
Acid Disassociation Constant [Ka] — stronger acids have a larger Ka value. |
|
pKa — negative log of the acid disassociation constant. |
|
|
Proton Concentration [pH] — a lower pH value indicates greater acidity, while a higher pH value indicates great basicity. |
|
|
Equilibrium Constant [Keq] — |

