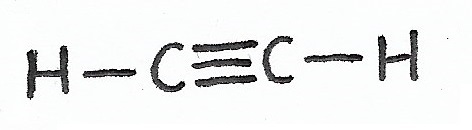► Science
► Physics
► Chemistry
Molecular Geometry
Introduction
Organic chemistry, also known as “carbon chemistry”, is chemistry involving organic — or “carbon-based” — molecules. All life on Earth can be considered an expression of complex carbon chemistry. However, since lots of non-living things are carbon-based as well, organic chemistry isn't merely limited to biochemistry. Organic chemistry also deals with polymers, petroleum products, and much more.
Orbital Hybridization
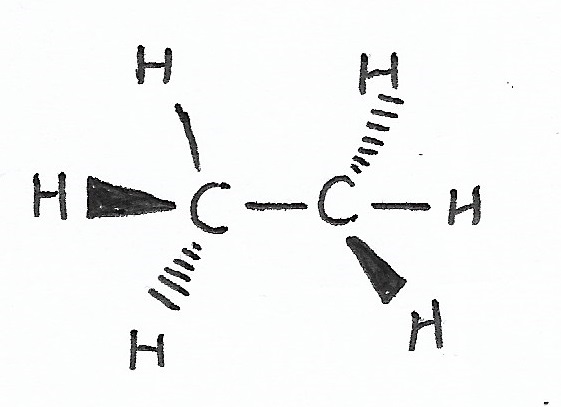 |
SP3 Hybridization / Tetrahedral Geometry (bond angle = 109.5°) — one 2s orbital with three 2p orbitals creating four hybrid orbitals.
|
 |
SP2 Hybridization / Trigonal Planar Geometry (bond angle = 120°) — one s orbital and two p orbitals.
|
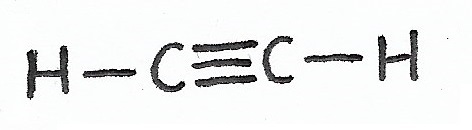 |
SP Hybridization / Linear Geometry (bond angle = 180°) —
|
Chirality
For more information, see chirality.
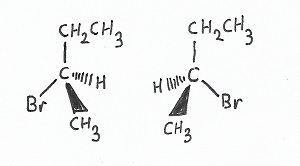 |
Rectus & Sinister Chirality — are ways of describing the ordering of attachment of function groups to a given chiral center (a carbon with four different groups attached to it, no two of which can be the same). If the function groups are ordered higher-to-lower in priority, or roughly so, in a clockwise or deosil orientation, the carbon is said to have rectus chirality. If the function groups are ordered higher-to-lower in priority, or roughly so, in a counterclockwise orientation, then the carbon is said to have sinister chirality.
|
|
Dextrorotatory & Levorotatory Chirality — when placed into a polarimeter or similar device, optically active molecules will rotate the plane-polarized light. Those substances that will rotate the plane clockwise or deosil are called dextrorotatory, while those that will rotate the plane counterclockwise or widdershins are called levorotatory. Dextrorotatory carbons almost always have rectus chirality, and levorotatory almost always have sinister chirality.
|
|
|