Acids & Bases
Introduction
Vital to the study of chemistry, especially organic chemistry and biochemistry, is understanding acid and base chemistry.
Famous acids include the amino acids.
| Acid | Base | |
| Arrhenius | H+ in H2O | OH— in H2O |
| Bronsted-Lowry | [H+] proton donor | [H+] proton acceptor |
| Lewis | electron pair acceptor | electron pair donor |
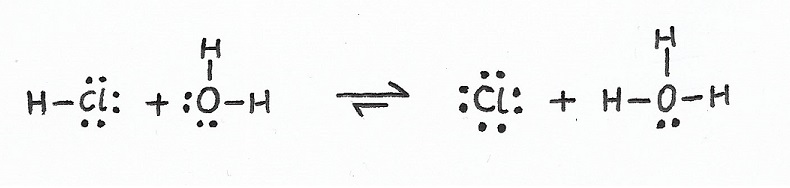
Conjugates

Important Units & Measurements
[Ka] |
Acid Dissociation Constant [Ka] — |
[pKa] |
–Log Acid Dissociation Constant [pKa] — this number can be used to calculate pH. |
[H+] |
Proton Concentration — |
[pH] |
–Log Proton Concentration — |
Strong Acids favor Reactants