H+
Introduction
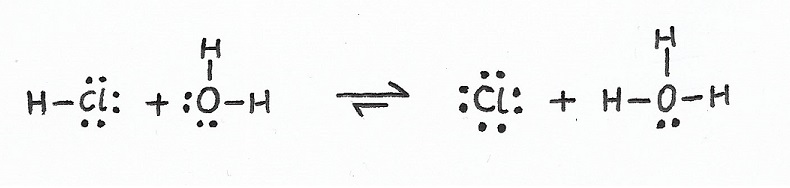
The concentration of free protons [H+] directly correlates with the strength of an acid.
—Log Proton Concentration [pH]
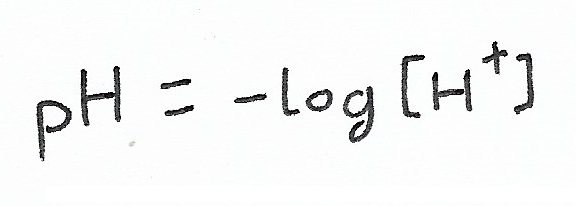
| Substance | [H+] concentration | scientific notation | pH |
| lemon juice | 0.01 | 1x10–2 | 2 |
| black coffee | 0.00001 | 1x10–5 | 5 |
| pure H2O | 0.0000001 | 1x10–7 | 7 |
| blood | 0.00000004 | 1x10–7.4 | 7.4 |
| sea water | 0.00000001 | 1x10–8 | 8 |
| baking soda | 0.000000000316227766 | 1x10–9.5 | 9.5 |
What if you know the pH of a substance, and want to know how that translates to proton concentration?
Just remember:

Knowing this, you can simply plug in your pH as your Y value, allowing X to represent your proton concentration.
Using pure water (pH=7), let’s first take the log positive by shifting the negative to the other side of the equation:

Having done, that, we can simply follow the “if log X = Y, then X = 10Y” logic:
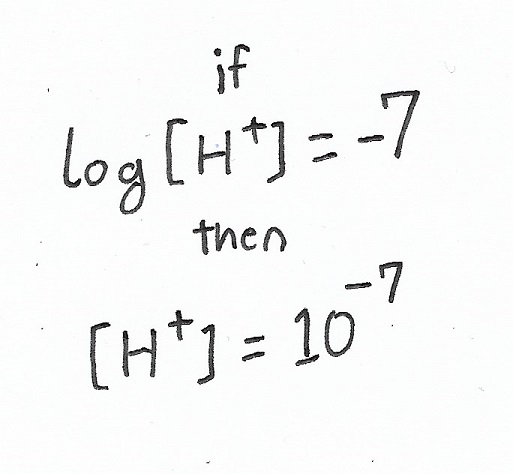
Thus, having run the calculation in reverse, we know that if pure water has a pH of 7, then its proton concentration must be 1x10-7 M.
Henderson-Hasselbalch equation
Another way to calculate pH, which is especially useful for buffer solutions:
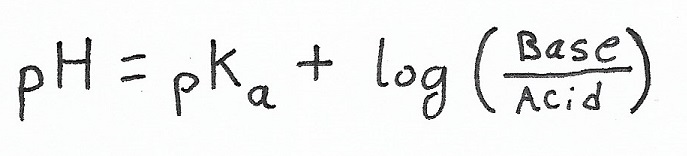
Strong Acids favor Reactants