pKa
—Log Acid Dissociation Constant [pKa]
 Methanoic Acid (Formic Acid) pKa = 3.75 |
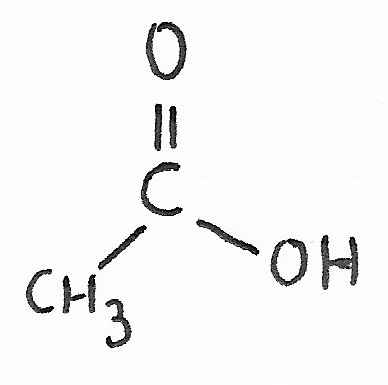 Ethanoic Acid (Acetic Acid) pKa = 4.76 |
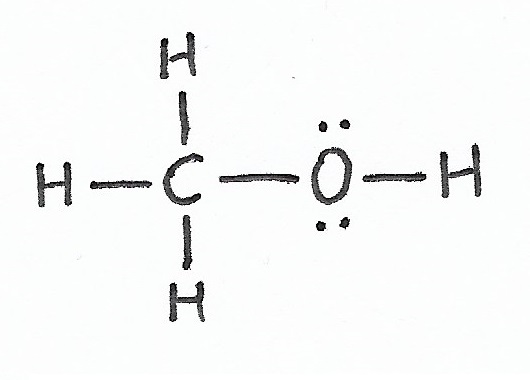 Methanol (Methyl Alcohol) pKa = 15.9 |
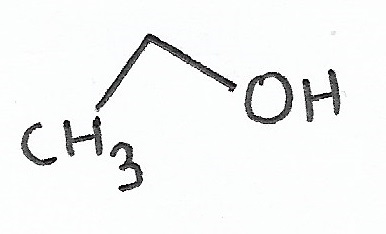 Ethanol (Ethyl Alcohol) pKa = 15.5 |
What if you know the pKa of a substance, and want to know how that translates to acid dissociation constant?
Just remember:


