Organic Chemistry
Introduction
Organic chemistry, also known as “carbon chemistry”, is chemistry involving organic — or “carbon-based” — molecules. All life on Earth can be considered an expression of complex carbon chemistry. However, since lots of non-living things are carbon-based as well, organic chemistry isn't merely limited to biochemistry. Organic chemistry also deals with polymers, petroleum products, and much more.
Organic Molecule Classes & Functional Groups
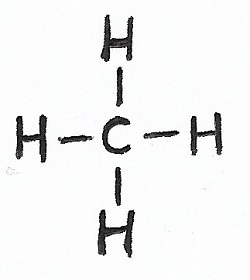 Methane |
Alkanes & Alkyl Groups — alkane molecules and alkyl functional groups are single-bonded hydrocarbons.
|
 Ethene |
Alkenes & Alkenyl Groups — characterized by double-bonds between carbons.
|
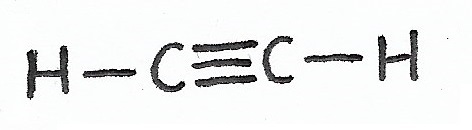 Ethyne |
Alkynes & Alkynyl Groups — alkyne molecules and functional groups have triple-bonds between carbon atoms. |
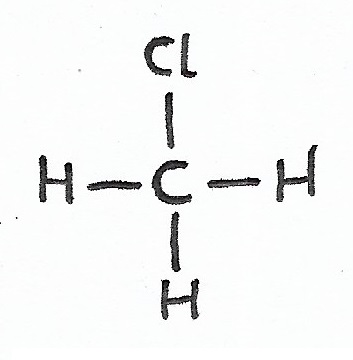 Chloromethane |
Alkyl Halides & Halide Groups — organic halide molecules, also known as halocarbons or alkyl halides, are carbon-based molecules that contain one or more halogens. |
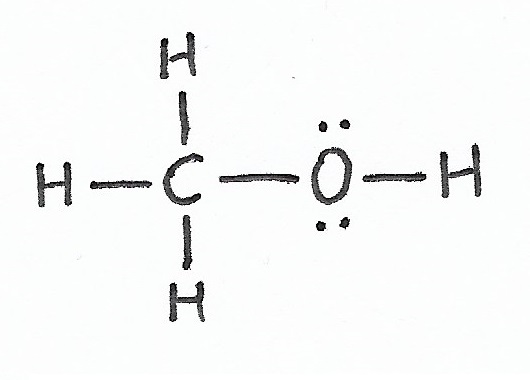 Methanol |
Alcohols & Hydroxyl Groups — alcohols are molecules that have one or more hydroxyl groups. |
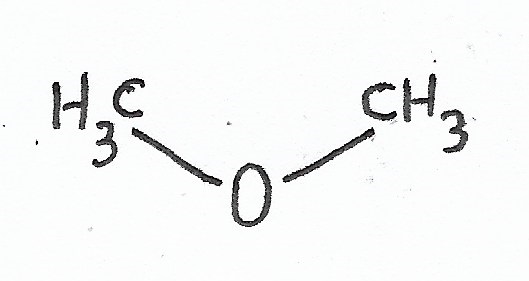 Dimethyl Ether |
Ethers — |
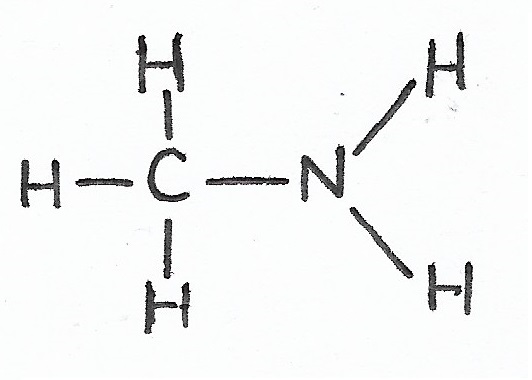 Methyl Amine |
Amines — |
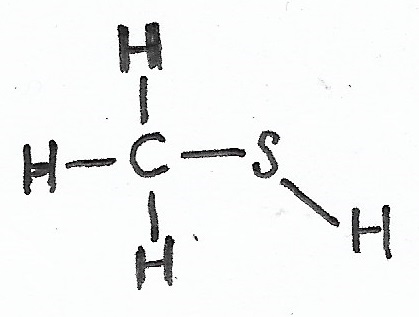 Methanethiol |
(Organic) Thiols — |
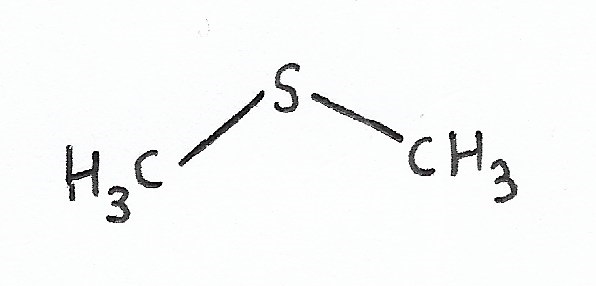 Dimethyl Sulphide |
(Organic) Sulphides — |
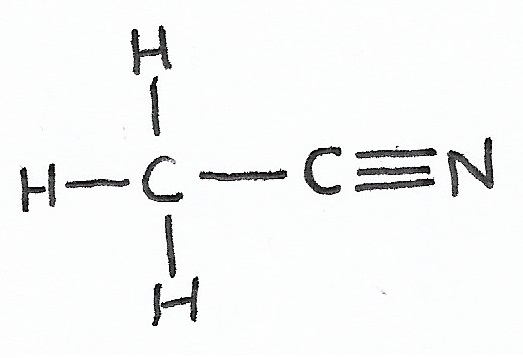 Acetonitrile |
Nitriles — |
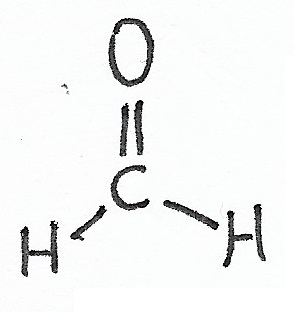 Methanal |
Aldehydes — the simplest aldehyde is methanal, also known as formaldehyde. |
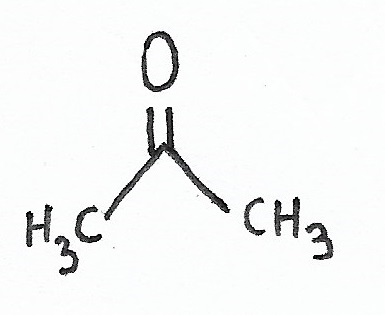 Propanone |
Ketones — the simplest ketone is propanone. |
 Methanoic Acid |
Carboxylic Acids — the simplest carboxylic acid is methanoic acid, also known as formic acid. |

