Configurational Stereoisomer
Introduction
Organic chemistry, also known as “carbon chemistry”, is chemistry involving organic — or “carbon-based” — molecules. All life on Earth can be considered an expression of complex carbon chemistry. However, since lots of non-living things are carbon-based as well, organic chemistry isn't merely limited to biochemistry. Organic chemistry also deals with polymers, petroleum products, and much more.
Geometric Stereoisomers
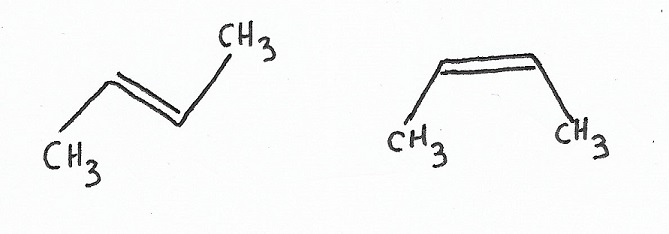 |
Cis & Trans Isomers — differ by the orientation of bonds to non-chiral stereocenters. The cis & trans prefixes can be thought of as notation used for “simple” cases of E/Z isomerism. |
 |
E & Z Isomers — differ by the orientation of bonds to non-chiral stereocenters. E/Z notation works similarly to cis/trans notation, and can work for those cases but, unlike cis/trans notation, is also used for more complex molecules where prioritization of function groups must be considered. |
Optical Stereoisomers
These are configurational stereoisomers that differ in the orientation of bonds to chiral stereocenters.
|
Diastereomers — differ in orientation around chiral centers. |
|
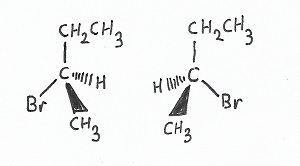 |
Enantiomers — are exact mirror-images of one another. |

