Enzyme
Introduction
Enzymes are proteins that act as biochemical catalysts. Enzymes speed up reactions.
Enzyme Kinetics:
Michaelis-Menten Model
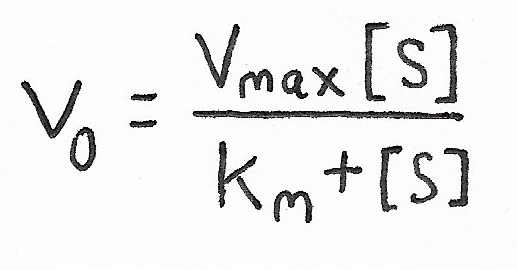
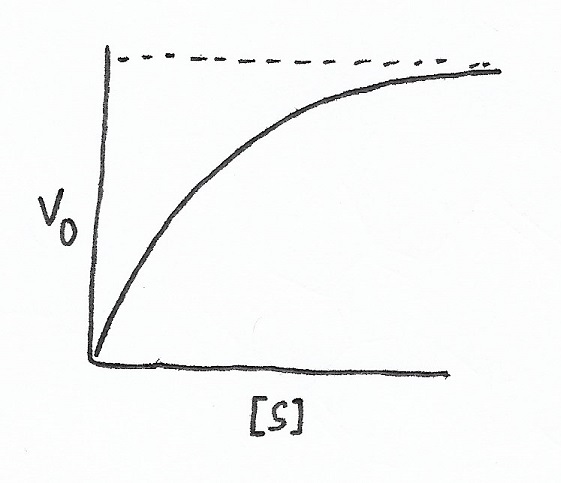 Hyperbolic V0 vs. [s] plot |
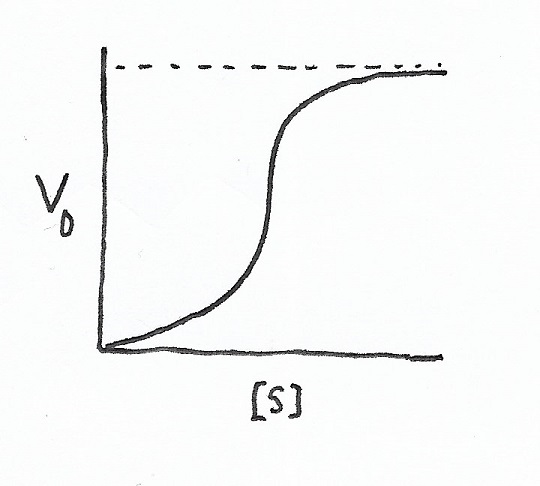 Sigmoidal V0 vs. [s] plot |
| Two versions of a traditional Michaelis-Menten velocity (V0) versus substrate concentration ([s]) plot. The first, hyperbolic, is the most common of the two. The second V0 vs. [s] plot is sigmoidal. Allosteric enzymatic activity control can result in a sigmoidal curve. | |
Enzymatic Activity Control:
Inhibitors
Competitive inhibitors — Try to bind at the same active site of an enzyme as the substrate, and thus compete for access to the active site. These therefore reduce their enzyme’s affinity for the substrate. Vmax does not change, and therefore nor does the Y-intercept, due to the fact that more substrate can overpower the effect of the comptetitive inhibitor.
Non-competitive inhibitors — these inhibit enzymes by altering their conformation. There is no reduction in affinity for the substrate. No change in X-intercept. Vmax is reduced, and cannot overcome this by adding more substrate. Change in Y-intercept.
Enzymatic Activity Control:
Allosteric Control
Allosteric effectors result in sigmoidal Michaelis-Menten (V0 vs. [s], or initial velocity vs. substrate) plots. Allosteric proteins typically comprise several subunits. An effector can either activate an enzyme or inhibit it. An effector causes a change in the enzyme molecule’s conformation.


